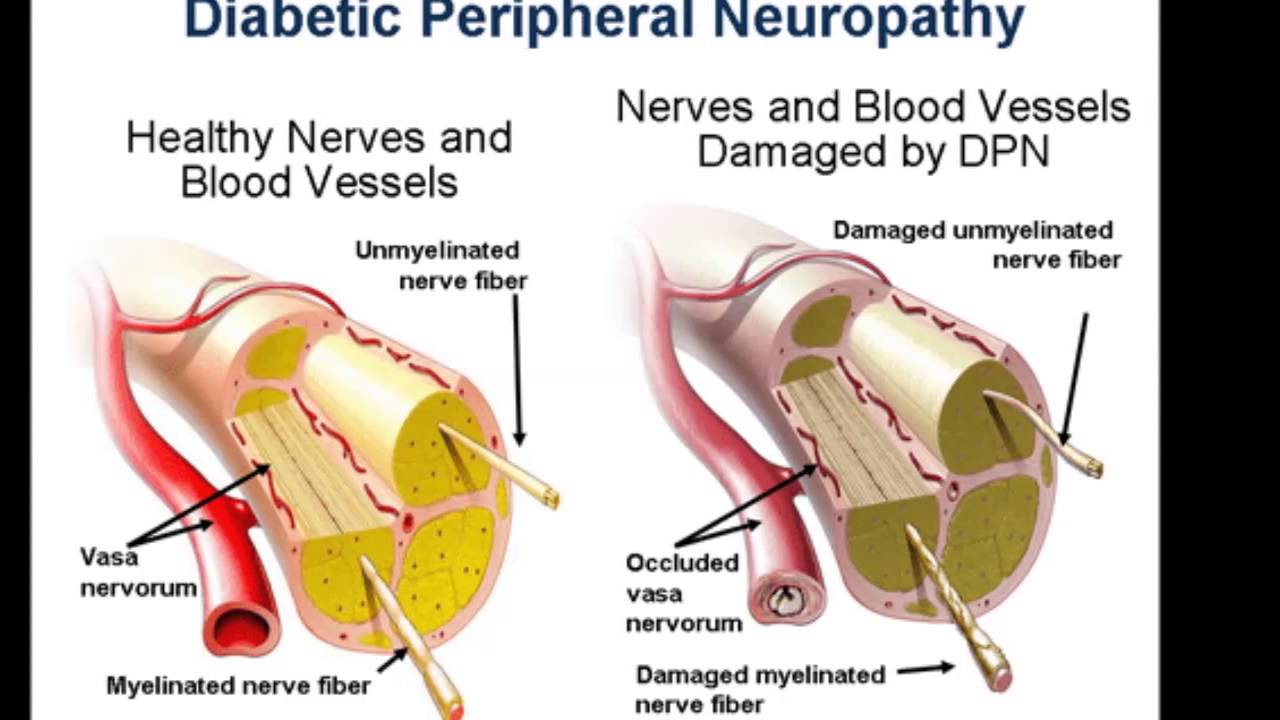
Not many people are aware of the medical condition that is known as Diabetic Neuropathy however more and more people are being diagnosed with having it, and if you have been recently diagnosed with Diabetic Neuropathy then you will need to start to take drug to help manage and control that condition.
The best drug you can take is the fast acting Gabapentin and one of the main reasons why many people who do have Diabetic Neuropathy will take that drug is that it is not only fast acting as mentioned but it is also a very low cost drug to purchase too.
Please do spend some time researching more information on Diabetic Neuropathy for when you do you will find that the very best course of action will be first to get that condition diagnosed by a Doctor and then the best treatment available will be by you then taking Gabapentin regularly to control that condition.
You will also be best advised to also spend a few minutes watching the following video that is going to explain to you more about Diabetic Neuropathy including the Types, Symptoms, Prevention and Treatment of Diabetic Neuropathy which you will certainly find very informative and educational too.
Also please do be aware that we do have a range of other articles and guides dotted around this website that will also give you additional information on a range of similar medical conditions that you may be suffering from or experiencing, so please do spend as much time as you like looking around our website as all of that information is free to access.
We also update our website continually with other related news stories and articles so do consider bookmarking this website and checking back regularly too.
If you do want to take Gabapentin to treat diabetic neuropathy then please do be aware there can be some side effects, and before you make a purchase of Gabapentin you will be best advised to find out what the side effect of Gabapentin when taking it to treat diabetic neuropathy, and if at any time you start to experience any of those side effects then please seek the advice of a Doctor or a medical professional.
There are going to be plenty of places online that you can buy Gabapentin, however when you make the very wise decision of using us as your official suppler of Gabapentin you are guaranteed of having the very lowest prices available to you and also as an approved stockist you will of course always be guaranteed of receiving genuine Gabapentin too.
To place an order right away simply click onto any of the order now links displayed on this site.
Gabapentin in the treatment of painful diabetic neuropathy: a placebo controlled, double blind, crossover trial [1]
Painful neuropathy is a common and disabling problem in patients with longstanding diabetes mellitus. Tricyclic antidepressant drugs and other chronic analgesics have been beneficial in some patients,1 but no agent successfully relieves pain in most patients and adverse effects often preclude their use in high doses.
Anecdotal reports suggest that gabapentin ameliorates pain associated with neuropathy and other neurological conditions with few side effects. We conducted a randomised, double blind, placebo controlled trial to study the effect of low dose gabapentin in patients with painful diabetic neuropathy.
We recruited 40 patients with painful diabetic neuropathy who had (1) diabetes for at least 6 months on a stable dosage of insulin or oral hypoglycaemic agent, (2) distal symmetric sensorimotor neuropathy as shown by impaired pin prick, temperature, or vibration sensation in both feet and absent or reduced ankle reflexes, and (3) daily neuropathic pain in the acral extremities, of at least moderate severity, for over 3 months that interfered with daily activity or sleep. Excluded were those with diabetes and chronic renal insufficiency, painful diabetic plexopathy, or lumbosacral polyradiculopathy, peripheral vascular disease, another painful condition, or other cause for neuropathy. Patients were randomly assigned to gabapentin (300 mg capsules) or placebo for 6 weeks (phase I) followed by a 3 week washout period and then crossover (phase II).
The dose of gabapentin or placebo was increased by one capsule every 3 days to a stable dosage of one capsule three times daily (900 mg/day) that was maintained throughout the remainder of the treatment period. The low dosage of gabapentin was chosen to minimise adverse effects that might compromise blinding. Treatment with stable dosages of non-steroidal anti-inflammatory agents or narcotics were permitted during the trial but patients discontinued all other chronic analgesic medications 3 weeks before study entry.
At the beginning and end of each treatment period, patients rated their level of pain over the preceding 24 hours on a 10 cm visual anologue pain scale (VAS), ranging from 0 (“no pain”) to 10 (“worst pain ever”). Present pain intensity (PPI, “rate how much pain you have at this moment,” using a similar 0–10 scale) and the McGill pain questionnaire (MPQ) were recorded at the initial and final visits of each treatment period.
At the end of each treatment period patients provided a global assessment of pain relief: none, mild, moderate, or excellent, as compared with the level of pain preceding each treatment period. The global assessment of pain relief was dichotomised (none/mild vmoderate/excellent) for purposes of analysis. The protocol was approved by the Institutional Review Board at St Elizabeth’s Medical Center and all patients gave written informed consent.
There were 31 men and nine women, with an average age of 62 years (SD 10.9 years, range 43–82 years). All but one had adult onset diabetes mellitus, with a mean duration of 14 years (SD 9.9 years, range 6 months-40 years). Ten had neuropathic pain limited to the feet, 19 had pain in the feet and legs, and 11 had pain in the feet, legs, and hands. The mean duration of neuropathic pain was 4 years (SD 3.5 years, range 4 months-15 years). Twenty five had previously used narcotics or other chronic analgesics to manage their pain.
Nineteen patients were randomised to the active drug and 21 to placebo during the first treatment period. The mean reduction in the MPQ score was 8.9 points with gabapentin compared with 2.2 points with placebo (p=0.03, two sample t test). There were no differences in the mean change of the VAS or PPI scores between gabapentin and placebo (table).
Fourteen patients reported moderate or excellent pain relief with gabapentin only, six with placebo only, and three with both; 17 reported none or mild relief after both treatments (p=0.11, McNemar’s test). There were no serious adverse events. Adverse effects were significantly more common with gabapentin (12 patients) compared with placebo (four patients, p<0.001, McNemar’s test). The most common side effects of gabapentin were drowsiness (six patients), fatigue (four), and imbalance (three). All adverse effects resolved promptly after discontinuation of the drug.
Comparison of mean change in pain scales between gabapentin and placebo
There was statistical improvement in only one of four end points, the MPQ score, with gabapentin compared with placebo. The MPQ is a valid, consistent, and reliable measure of subjective pain experience, and usually correlates with other measures of pain intensity, including the VAS and PPI scales.4 We designed the study to have an 80% power to detect a 20% reduction in pain scores, reflecting a modest but clinically important improvement.
The mean change of the VAS and PPI scales and the patient’s global assessment of pain relief were not significantly different from placebo. We used a crossover design because of its statistical efficiency, but the MPQ and VAS scores did not return to baseline after crossover in patients who received gabapentin in phase I (the washout period was inadequate); therefore, we may have underestimated improvement with gabapentin in the VAS scale that may have been detected using a parallel group design.
Furthermore, a limitation of our study was that quantitative measures (for example, nerve conduction studies, quantitative sensory thresholds) were not used to further characterise the type of neuropathy. Because of the heterogeneous nature of neuropathic pain in our study patients, we may not have identified a subset of patients who improved with gabapentin. Alternatively, the dosage of gabapentin may have been too low to induce analgesia in patients with painful diabetic neuropathy, although similar regimens have been reported to be effective in patients with other painful conditions.
The results of this study suggest that gabapentin is probably ineffective or only minimally effective for the treatment of painful diabetic neuropathy at a dosage of 900 mg/day.